
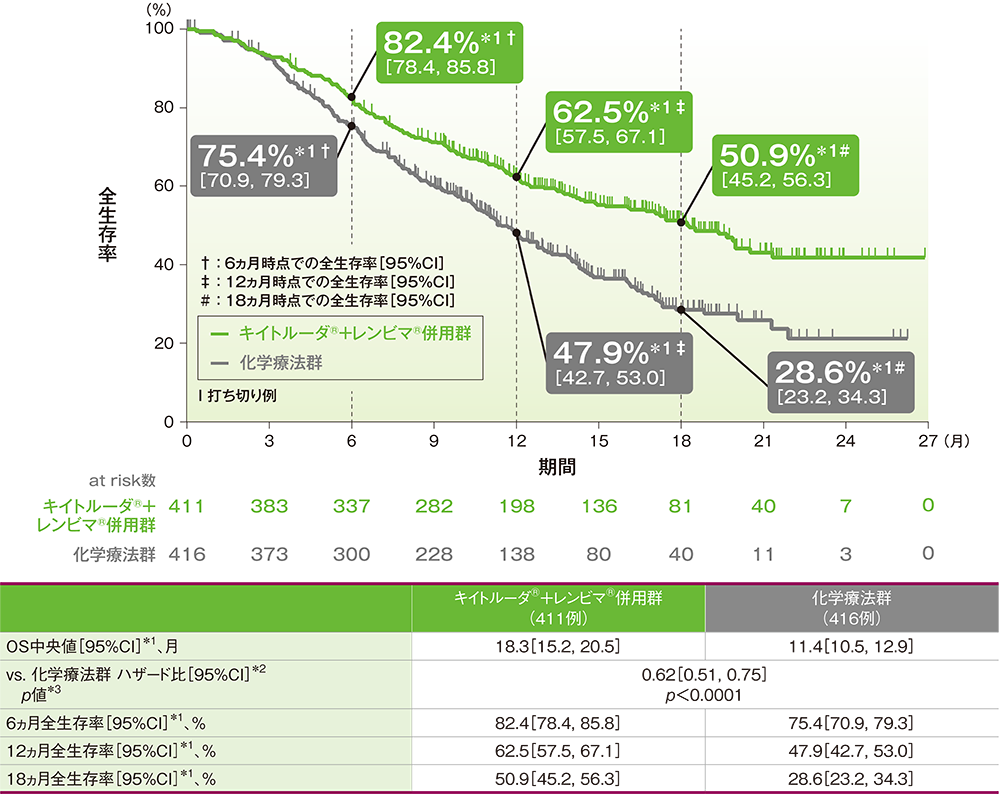
The New England Journal 2022 contains results for the all-comer population, including the mismatch repair deficient (dMMR) patient population for which Lenvima plus Keytruda, which has not yet been approved in the US. "KEYNOTE-775/Study 309 is an important phase 3 study that supported recent approvals of Keytruda plus Lenvima for certain types of advanced endometrial carcinoma in the US and other countries around the world, where it became the first immunotherapy and tyrosine kinase inhibitor combination approved for these patients." Speaking on the results of the trial, Dr Gregory Lubiniecki, vice president, Oncology Clinical Research at Merck Research Laboratories, commented: "While rates of endometrial carcinoma continue to rise globally, patients with advanced or recurrent disease have limited options available to them once the disease progresses following platinum-based chemotherapy.

The results presented demonstrated that the combination of Lenvima plus Keytruda showed statistically significant improvements regarding the dual primary endpoints of progression-free survival and overall survival, compared to chemotherapy. Included in the journal publication was previously recorded data, originally presented at the virtual Society of Gynecologic Oncology (SGO) 2021 Annual Meeting on Women's Cancer. The study indicated that the chemotherapy used was down to the physician's choice of either paclitaxel or doxorubicin. The crucial study assessed the combination of Lenvima (lenvatinib) – a multiple receptor tyrosine kinase inhibitor administered orally as a pill, discovered by Eisai – with Merck’s anti-PD-1 therapy Keytruda (pembrolizumab) versus chemotherapy for patients with advanced, metastatic, or recurrent endometrial carcinoma following one prior platinum-based regimen.

Merck – known as MSD outside of the US and Canada – announced in the New England Journal 2022 edition a publication of results from the phase 3 Study 309/KEYNOTE-775 trial.


 0 kommentar(er)
0 kommentar(er)
